View Procedure
| Procedure Name | Import Permit for Medicines and Allied Substances |
|---|
| Description |
|
Category
|
Medicines and Allied Substances Import Permit
|
|
Responsible Agency
|
Zambia Medicines Regulatory Authority
Address:Tuleteka Road, Off Makishi Road
P.O. Box 31890
Lusaka , Zambia
Phone : +260 211 220 429
Email : pharmacy@zamra.co.zm
|
|
Legal base of the Procedure
|
The Medicines and Allied Substances Act,2013 (MASA)
|
| Fee |
Application Fee ZMW 100
|
Preclearance Fees for Quality Assurance (QA) of imports for commercial consignments, Government ministries departments, Programs projects, and similar institutions
|
1.5% of FOB invoice value
|
|
Preclearance Fees for Quality Assurance (QA) of imports for unregistered medicines and Allied substance for commercial consignments, Government Ministries Departments, Programs projects and Similar institutions
|
5% of FOB invoice value
|
|
Preclearance Fees for Quality Assurance (QA) of imports for Donations
|
1% of FOB invoice value
|
|
Preclearance Fees for Quality Assurance (QA) of imports for Active Pharmaceutical Ingredients (API), Bulk finished products and intermediates
|
1% of FOB invoice value
|
|
Required Documents
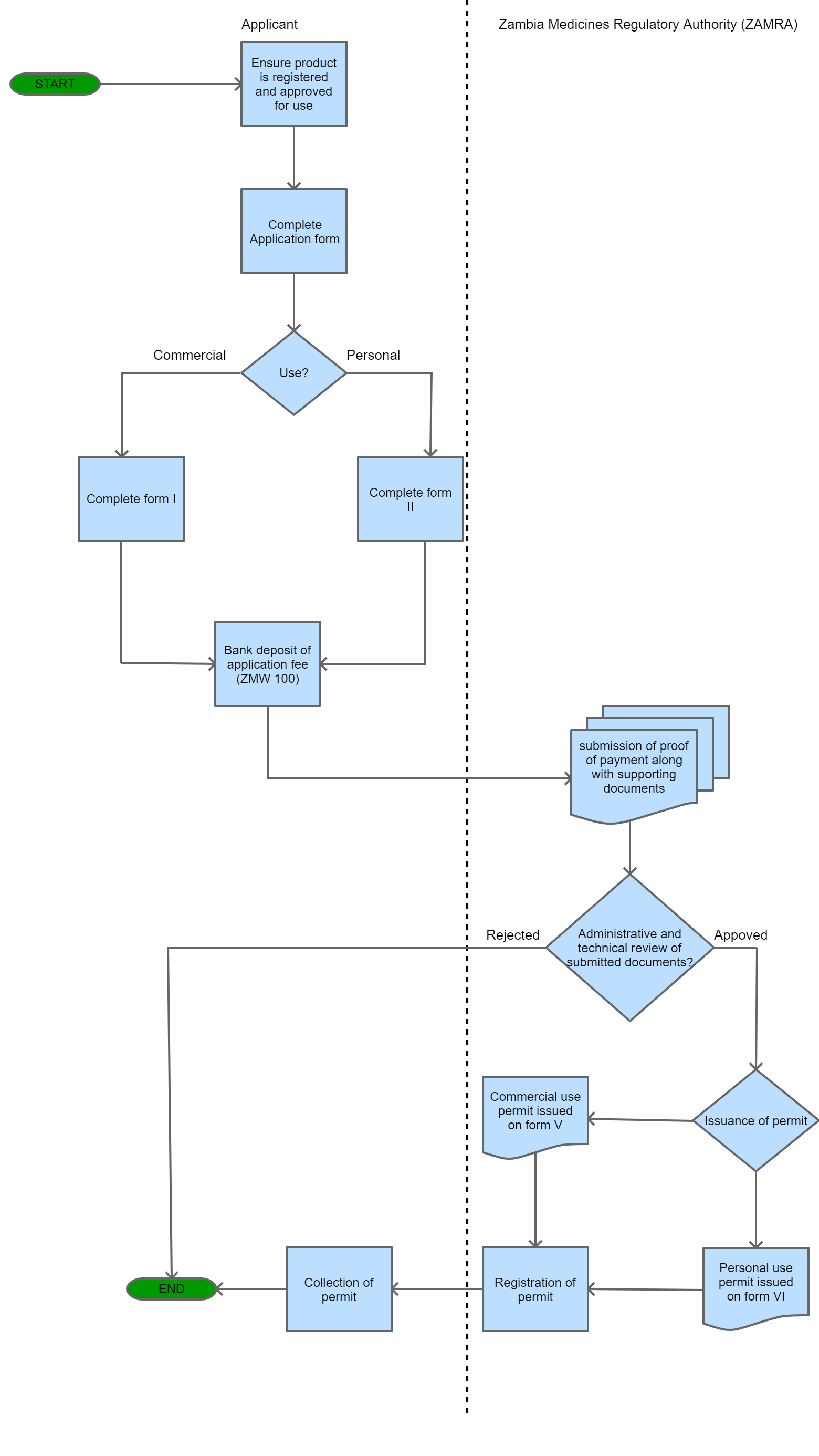
Process Steps
|
Step 1
|
Importer proceeds to complete the application form. That is Form I which is found in the Schedule if importing for commercial use. Form II is used if a person is importing for personal use. The trader must also gather all supporting documentation.
|
|
Step 2
|
Importer pays the prescribed application fees into a bank, who will then issue proof of payment. This must be presented to the relevant accounts department at ZAMRA who will then issue a receipt that must be submitted along with the application and supporting documents.
|
|
Step 3
|
The approving section conducts an administrative and technical review of the application and if they have no objection to the application, then it is returned to the receiving officer.
|
|
Step 4
|
The issuing section processes the application and then issues the Import Permit on the prescribed Form V if the permit is for commercial use or Form VI if the permit is for personal use.
|
|
Step 5
|
The receiving officer then registers the import permit on the prescribed Form XI.
|
| Step 6 |
The trader collects the import permit and may proceed to import the products into the country. |
|
|---|
| Category | Import |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures




