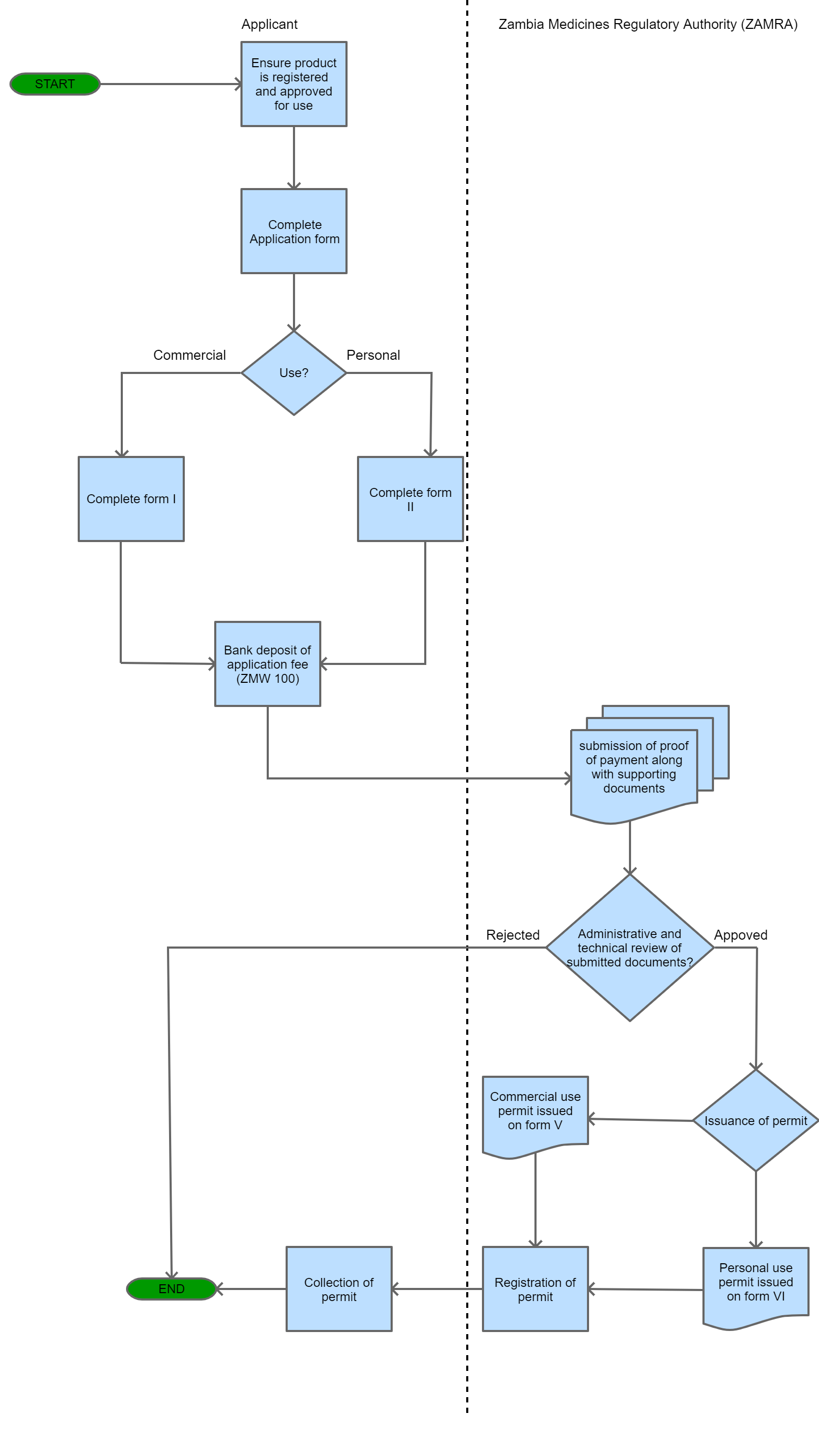
View Procedure
| Procedure Name | Export Permit for Medicines and Allied Substances | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required Documents
Process Steps
| |||||||||||||||||||||||||||||||||||
| Category | Export |
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Importation or Exportation of Medicines Permit or Medicine or Allied Substance Form I | Application for Importation or Exportation of Medicines for Commercial use | 07-11-2019 | 07-11-2019 | ||
| Application for Importation or exportation of Medicines and Allied Substances for personal use Form II | Application for Importation or exportation of Medicines and Allied Substances for personal use | 07-11-2019 | 07-11-2019 |
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Export Permit requirement for Medicines and Allied Substances | Permit Requirement | No person shall export any medicine or allied substance without an export permit issued by Zambia Medicines Regulatory Authority. | An export permit is required for the exportation of medicines and allied substances. | The Medicines and Allied Substances Act, 2013 | 09-09-9999 | Good |