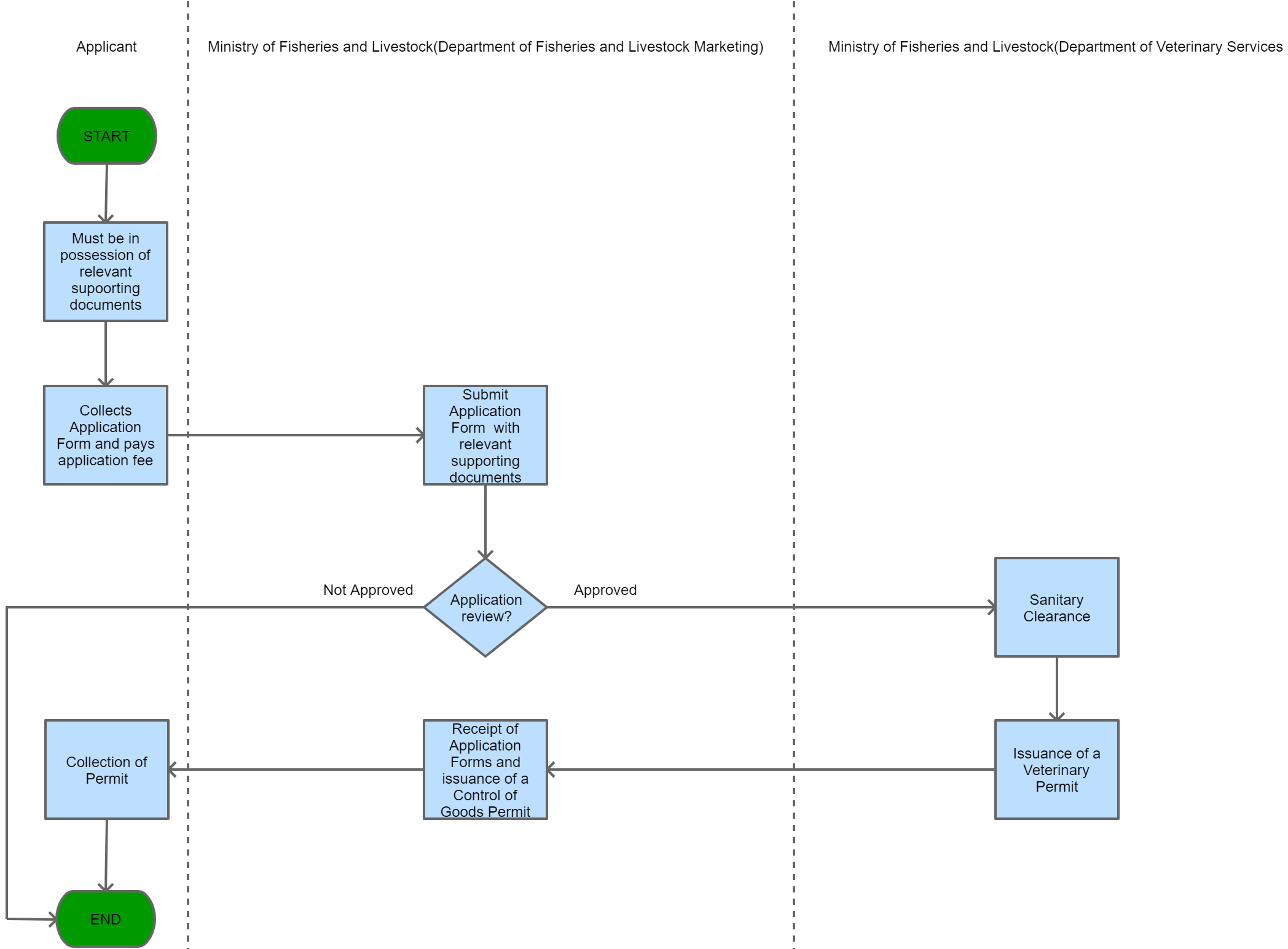
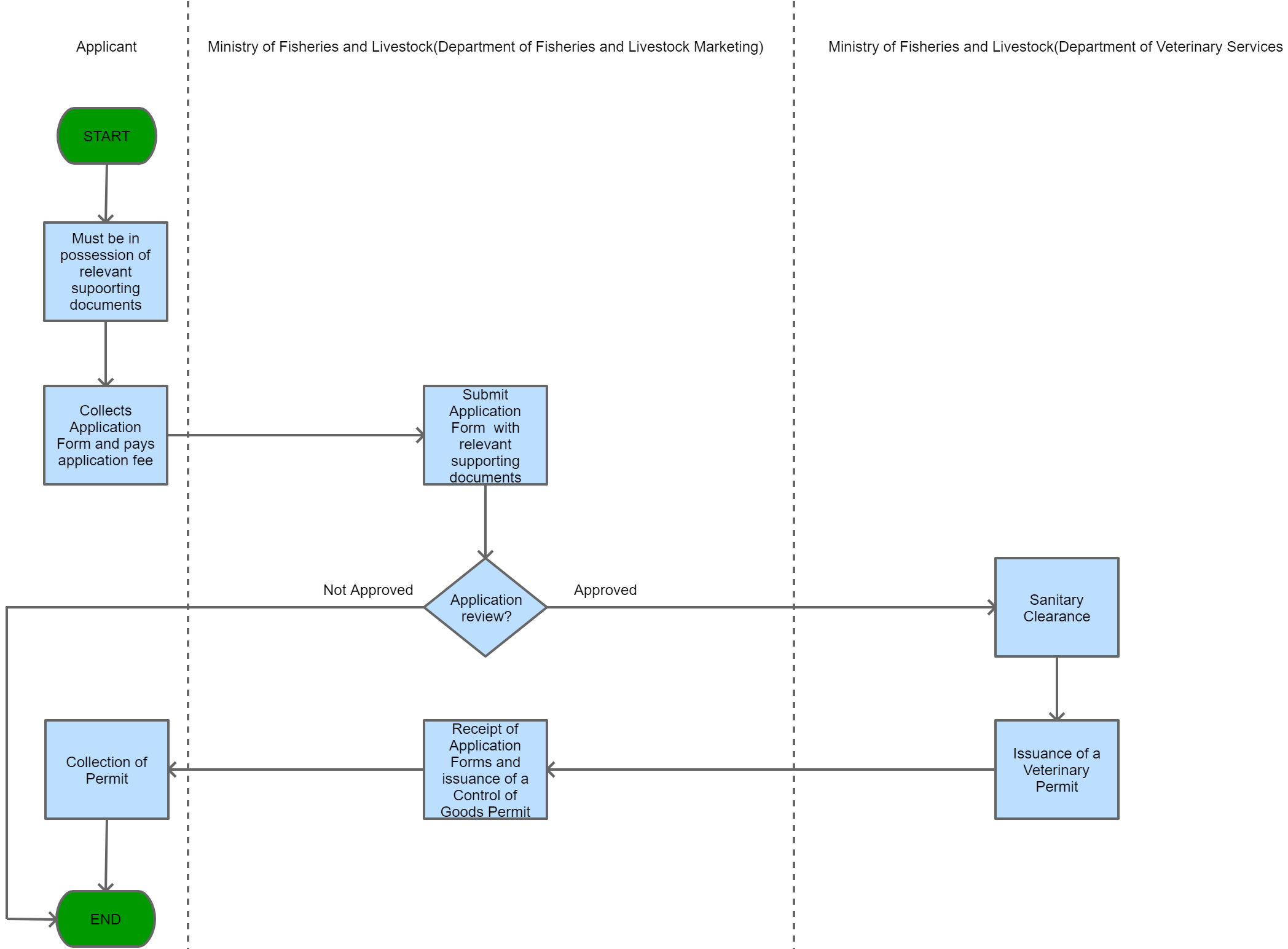
View Procedure
| Procedure Name | Permit requirement for importation of Virus, vaccine, Serum or an Analogous product for treatment of Animal Diseases | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required Documents
Process Steps
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Category | Import |
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Application for a Fisheries and Livestock Import Permit | Application for a Fisheries and Livestock Import Permit | 08-11-2019 | 08-11-2019 |
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Permit requirement for importation of Virus, vaccine, Serum or an Analogous product for treatment of Animal Diseases | Permit Requirement | In order to import into Zambia a virus, vaccine, serum or an analogous product used for the purpose of diagnosis or treatment of any animal disease a permit in writing issued by the Ministry of Fisheries and Livestock is required. | No virus, vaccine, serum or an analogous product used for the purpose of diagnosis or treatment of any animal disease can be imported into Zambia without a permit in writing issued by the Ministry of Fisheries and Livestock. | The Animal Health Act, 2010 | 09-09-9999 | Good |